TMS FSMA 204 Compliance Automation: The 72-Hour Configuration Protocol That Prevents FDA Response Failures

You've spent months hearing about the original January 2026 deadline for FSMA 204 compliance. The enforcement deadline for the Food Traceability Rule has been extended from January 20, 2026 to July 20, 2028. But while everyone's processing this 30-month extension, TMS teams are stuck in a dangerous paralysis: Do you pause configuration work or accelerate it?
Your TMS FSMA 204 compliance configuration can't wait another two years. Here's why the smart move is building your 72-hour response protocol now, before the rush hits and before your suppliers get overwhelmed with compliance demands.
The Deadline Extension Creates Operational Chaos, Not Clarity
The core requirements remain unchanged, and market-driven demands are creating higher standards than FDA mandates. Some retailers already require traceability data within two hours—not 24—which means upstream suppliers must be technologically ready well ahead of regulatory deadlines.
Your procurement team knows this reality. Buyers are using traceability as a procurement filter. Industry procurement trends show that companies are adding "FSMA 204 ready" as a qualification criterion in RFPs dated for mid-2026. While the FDA gave you until 2028, your customers didn't get that memo.
Meanwhile, food manufacturers and distributors that handle items on the FTL must still be prepared to provide KDEs and lot-level tracking data within 24 hours upon request. This 24-hour response requirement stays in effect regardless of the extended deadline. You need systems that can handle both routine operations and emergency FDA requests.
Critical Tracking Events Your TMS Must Capture Automatically
FSMA 204 mandates that entities involved in manufacturing, processing, packing, or holding foods listed on the Food Traceability List (FTL) maintain detailed records of Key Data Elements (KDEs) associated with specific Critical Tracking Events (CTEs).
Your TMS needs to capture five core CTEs without manual intervention:
- Receiving events: Product source location, harvest/production dates, traceability lot codes from your suppliers
- Shipping events: Customer destination, shipment identification numbers, carrier details
- Transformation events: When products change form during processing (mixing, packaging, combining lots)
- Creation events: Initial processing or manufacturing that creates traceable lots
- Depletion events: When traceable products leave your facility for retail or further processing
The configuration challenge? Your TMS needs to integrate with WMS and ERP systems to capture this data at the transaction level. TMS can integrate with other supply chain management systems, such as Warehouse Management Systems (WMS) and Enterprise Resource Planning (ERP) systems, to provide a comprehensive view of the entire supply chain. This integration allows for seamless data flow between different functions, leading to improved coordination and decision-making across the organization.
Modern platforms like Cargoson, Descartes, Oracle TM, and E2open all handle automated KDE capture, but configuration varies significantly. Your receiving workflows need to automatically pull traceability lot codes from inbound EDI transactions, not rely on manual data entry that creates compliance gaps.
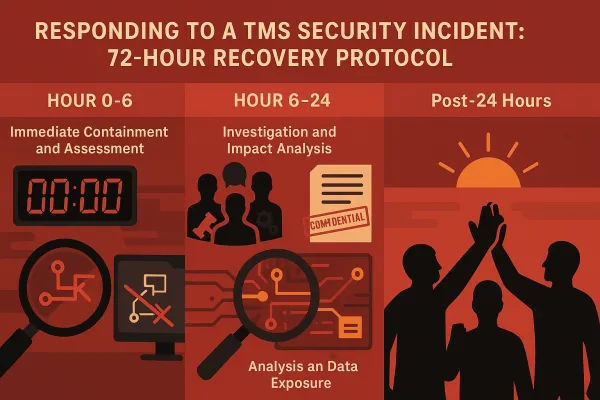
The 72-Hour Response Protocol That Prevents FDA Failures
The FDA requires records within 24 hours, but smart operators configure 72-hour response protocols. Here's why: when your primary system fails, you need automated backup procedures that prevent compliance failures.
Configure your TMS with these fail-safe mechanisms:
Automated record retrieval: When FDA sends a traceability request, your system should automatically generate the required KDEs without human intervention. Configure APIs that pull data from multiple systems (ERP, WMS, TMS) and format it according to FDA specifications.
Backup data storage: Your TMS should maintain traceability records in both primary and secondary locations. If your main database experiences downtime, backup systems need to respond within hours, not days.
Supplier verification protocols: Configure automated checks that verify incoming supplier data contains required KDEs before accepting shipments. You can have the most sophisticated traceability system in the world, but if your tomato supplier can't provide proper harvest location data and traceability lot codes, you're non-compliant. Supplier onboarding has proven to be one of the biggest hurdles for enterprise organizations.
Platforms like Cargoson, MercuryGate (now Infios), and SAP TM offer different approaches to automated compliance workflows. Some use webhook-triggered responses, others rely on scheduled batch processes. The key is testing your response time under load—can your system handle simultaneous FDA requests for multiple product lots?
TMS-ERP Integration Points for Automated KDE Capture
The biggest configuration challenge isn't within your TMS—it's the integration points between TMS, ERP, and WMS systems. A TMS system works in conjunction with other software as part of a broader supply chain management system – and most offer ERP and warehouse management system (WMS) integration. Each software system below performs a specific function; when integrated, they form a digital tripod that supports delivery of the end-to-end process.
Critical integration configurations:
Lot code synchronization: Configure your TMS to automatically receive traceability lot codes from ERP during order creation. This prevents manual entry errors and ensures consistency across systems.
Facility identification mapping: Your TMS needs to automatically map internal facility codes to FDA-compliant location identifiers. This requires configuration that translates between your internal naming conventions and regulatory requirements.
Document flow automation: Configure EDI transactions (856 ASNs, 810 invoices) to include required KDEs automatically. Your trading partners need this data without requesting it separately.
Systems like Blue Yonder, Manhattan Active, nShift, and Cargoson handle these integrations differently. Some require custom API development, others offer pre-built connectors. The configuration complexity depends on your ERP system—SAP implementations typically require more custom work than simpler ERP platforms.
Supplier Compliance Verification That Actually Works
FSMA 204 compliance is particularly vulnerable because your success depends on suppliers changing how they do business with you. Your TMS configuration needs to handle supplier compliance verification automatically, not rely on manual processes that break under pressure.
Configure these supplier workflows:
Onboarding validation: New suppliers can't ship until their systems pass traceability tests. Configure your TMS to require test transactions that demonstrate KDE compliance before approving supplier relationships.
Real-time compliance monitoring: Configure alerts when suppliers send shipments missing required KDEs. A TMS ensures compliance with regulatory, quality, and security standards, plus automatic documentation, tracking, and transparency. Your system should flag non-compliant shipments before they reach your facility.
Performance scoring: Configure supplier scorecards that track traceability compliance rates. Suppliers with consistent compliance failures need intervention or replacement.
Platforms like Transporeon, Alpega, and Cargoson offer different approaches to multi-supplier compliance verification. Some focus on EDI validation, others emphasize portal-based data collection. The key is choosing an approach that scales—you can't manually verify every supplier shipment when volume increases.
Exception Handling Configuration for Non-Compliant Shipments
Your TMS needs to handle compliance exceptions automatically. When shipments arrive missing KDEs, you need instant alerts and predefined workflows that prevent compliance failures.
Configure these exception scenarios:
Missing traceability lot codes: When suppliers send shipments without proper lot identification, configure your TMS to quarantine products until compliance data arrives. This prevents non-compliant products from entering your supply chain.
Temperature excursions: For temperature-sensitive FTL products, configure real-time alerts when monitoring devices detect deviations. Your TMS should automatically notify quality teams and trigger corrective actions.
Supplier system failures: When supplier systems go offline, configure backup procedures that maintain traceability without disrupting operations. This might involve temporary manual processes with automated validation once systems recover.
Solutions like FreightPOP, 3Gtms/Pacejet, and Cargoson handle compliance exceptions differently. Some use rule-based workflows, others rely on machine learning algorithms. Configure the approach that matches your team's capabilities—sophisticated AI-driven systems require different skill sets than rule-based alternatives.
Post-Implementation Validation and Audit Readiness
Configuration work isn't complete until you've validated your 24-hour FDA response capability. This requires testing scenarios that simulate real audit requests.
Test these validation scenarios:
Mock FDA requests: Generate test requests for specific product lots and measure response times. Your TMS should produce complete traceability records within hours, not days.
Cross-system data accuracy: Verify that KDEs match across TMS, ERP, and WMS systems. Inconsistencies create audit failures even when individual systems work correctly.
Supplier integration stress tests: Test compliance workflows when multiple suppliers send data simultaneously. Your integration points need to handle peak loads without failures.
Enterprise platforms like Oracle TM, SAP TM, and Cargoson offer different audit capabilities. Some provide built-in compliance dashboards, others require custom reporting development. The key is demonstrating audit readiness before FDA enforcement begins.
Companies achieving early compliance see competitive advantages beyond regulatory requirements. While others struggle with last-minute implementations, early movers secure supplier relationships, win customer contracts, and build operational resilience that extends beyond FSMA 204 requirements.
Start your TMS FSMA 204 configuration now. The 30-month extension gives you time to build robust systems, not permission to delay. Your competitors are already moving—the question is whether you'll lead the market or scramble to catch up.